Published reports in book form

Pfizer Documents Analysis Reports
Still available as a PDF on dailyclout.io and also on Amazon as a Kindle Book
The Pfizer Papers: Pfizer’s Crimes Against Humanity – Amazon hardcopy
The Pfizer Papers. Ed Naomi Wolf and Amy Kelly. Skyhorse Publishing 2024 ISBN 10 1648210376
The WarRoom/DailyClout Pfizer Documents Analysts. Pfizer Papers: Pfizer’s Crimes Against Humanity. Edited by Naomi Wolf and Amy Kelly, WarRoom Books, New York, 2024. ISBN: 9781648210372
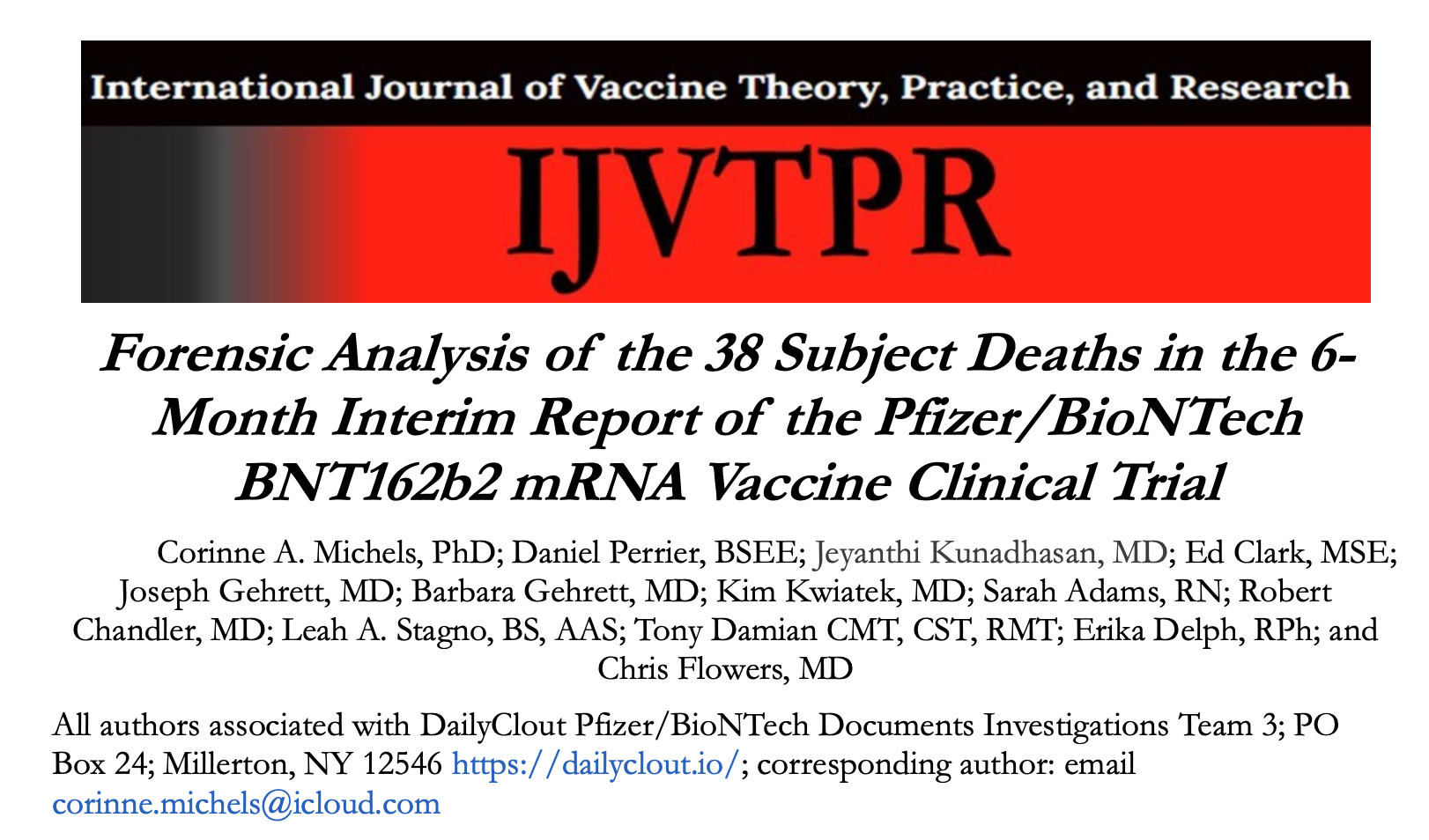
https://ijvtpr.com/index.php/IJVTPR/article/view/86
The analysis reported here is unique: it is the first study of the original data from the Pfizer/BioNTech BNT162b2 mRNA vaccine clinical trial (C4591001) to be carried out by a group unaffiliated with the trial sponsor. Our study is a forensic analysis of the 38 trial subjects who died between July 27, 2020, the start of Phase 2/3 of the clinical trial, and March 13, 2021, the end date of the official 6-Month Interim Report. Phase ⅔ of the trial involved 44,060 subjects who were equally distributed into two groups and received dose 1 of either the BNT162b2 mRNA vaccine or a placebo consisting of a 0.9% normal saline solution. At week 20, when the BNT162b2 mRNA vaccine received Emergency Use Authorization from the US FDA, subjects in the
placebo arm were given the option to receive the BNT162b2 vaccine and switch to the vaccinated group. Of the reported 20,794 unblinded placebo subjects, 19,685 received at least one dose of BNT162b2 vaccine.
Surprisingly, a comparison of the number of subject deaths per week during the 33 weeks of this study found no significant difference between the number of deaths in the vaccinated versus placebo arms for the first 20 weeks of the trial — the placebo-controlled portion of the trial.
After week 20, as subjects in the placebo group were unblinded, and after the majority of them received a BNT162b2 injection, deaths among those
sticking with the placebo slowed and eventually plateaued. Deaths in the BNT162b2 vaccinated subjects continued at the same rate.
Our analysis reveals inconsistencies between the subject data listed in the 6-Month Interim Report and in publications authored by Pfizer/BioNTech trial site administrators. Most importantly, we found evidence of an over 3.7-fold increase in number of deaths due to cardiac events in the BNT162b2 vaccinated individuals compared to those who received only the placebo. Delayed reporting of the subject deaths into the Case Report Form obscured the cardiac adverse event signal and allowed the Pfizer/BioNTech Emergency Use Authorization to proceed unchallenged.
Write ups and interviews:
- https://www.theepochtimes.com/health/researchers-find-pfizer-excluded-clinical-trial-deaths-from-fda-covid-vaccine-eua-request-5511880
- https://www.trialsitenews.com/a/volunteer-study-groups-investigation-findings-may-portend-big-trouble-for-pfizer-f68896a5
- https://www.trialsitenews.com/a/intensive-interrogation-of-pfizer-bnt162b2-trial-dataappears-to-be-research-malfeasance-cover-up-ed0b9bdd
Interview of Prof C Michels and Dan Perrier about the paper –

Undisclosed Deaths in the Pfizer mRNA COVID-19 Vaccine Trial: Will There Be Accountability?
Jeyanthi Kunadhasan, M.D. Corinne A. Michels, Ph.D.
Journal of American Physicians and Surgeons Volume 30 Number 4 Winter 2025

Click the link for a FREE download of the PDF
Write ups:
CITATIONS
Myocarditis after SARS-CoV-2 infection and COVID-19 vaccination: Epidemiology, outcomes, and new perspectives
DOI: 10.61577/ijcri.2025.100001
M. Nathaniel Mead, Jessica Rose, William Makis, Kirk Milhoan, Nicolas Hulscher and Peter A. McCullough (April 2025)
SUBSTACKS
Leah Stagno
BioTechBabe Substack
- Kim Kwiatek – Doc of Last Resort‘s Newsletter
- Dr Chris Flowers MD – A Bunch of Flowers
- Prof Corinne Michels – Science Rules
- Dr Jeyanthi Kunadhasan – JKun’s Substack
- David Shaw – Publius
- Blue Butterfly
- Leah – Biotech Babe