The Emergency Use Authorization (EUA), the Food and Drug Administration (FDA), the Centers for Disease Control (CDC) and Pfizer
Understanding the Pfizer Clinical Trial and the reasons why analysis has shown real issues

The Food and Drug Administration

Pfizer
“The “Sponsor” of the Clinical Trial
The Hidden Deaths – Hidden Autopsies – and Lost to Follow-up
A Forensic Analysis of the Pfizer Clinical Trial – IJVTPR Paper Oct 2023
As described by Dr Jeyanthi Kunadhasan
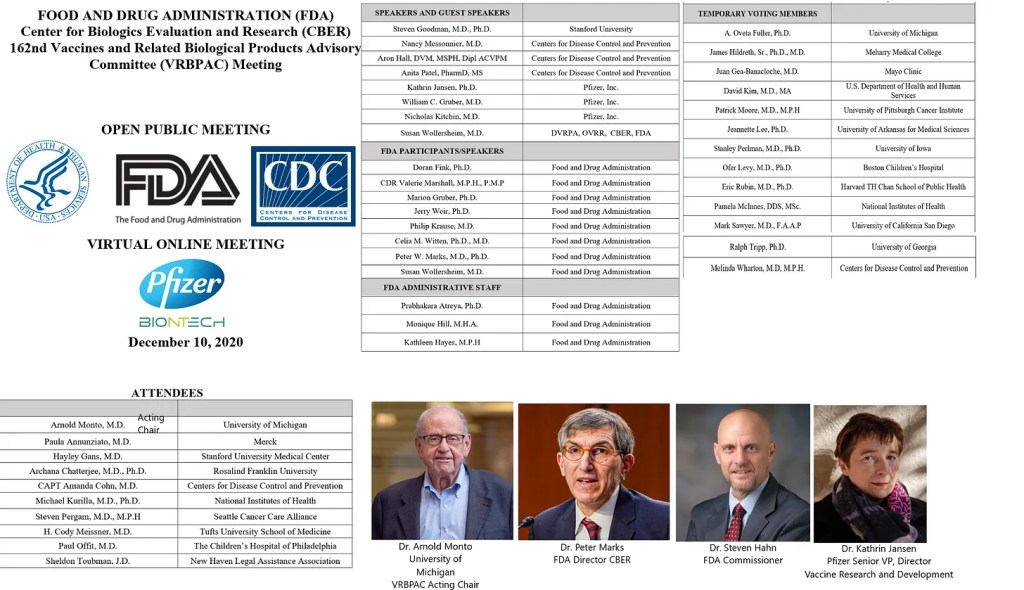
The December 10, 2020 FDA VRBPAC Meeting that approved the Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 Vaccine (also known as BNT162b2)
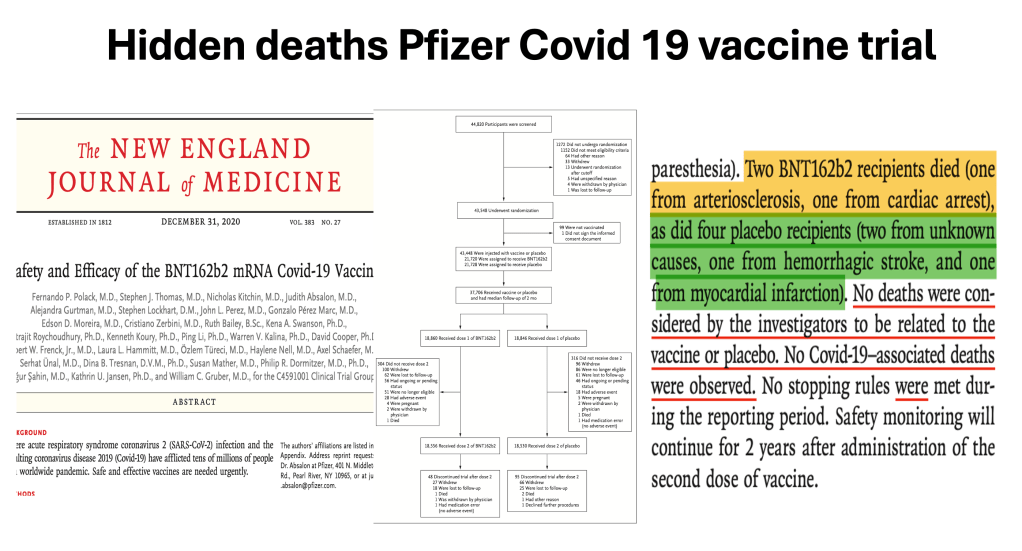
There were two published papers in the New England Journal of Medicine regarding the trial
The first was Polack et al, published 12/10/2020 the day AFTER approval of the EUA
The second one was Thomas et al, published 9/15/21 based on the six month data from the trial
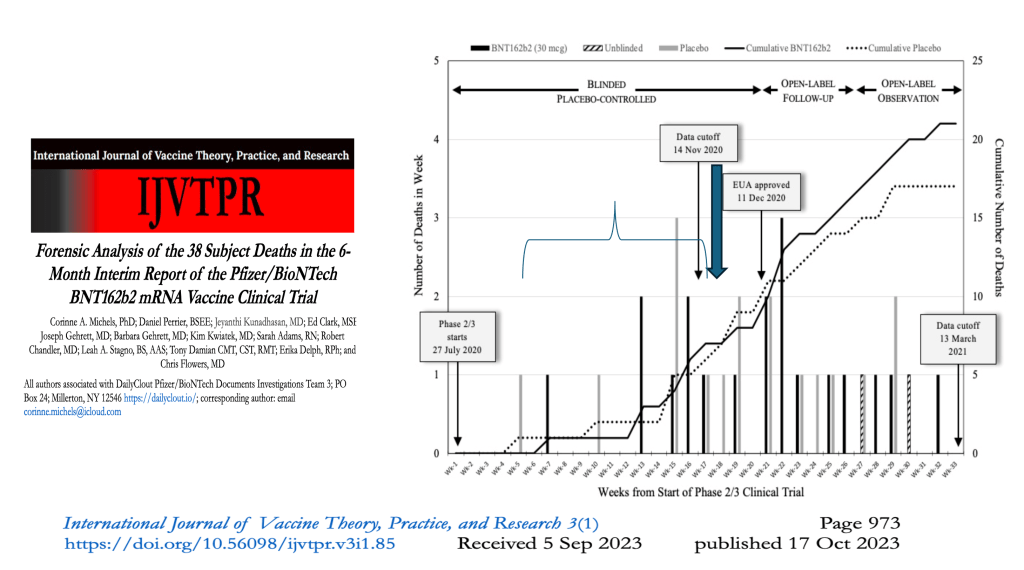
Our comprehensive analysis of the entire Pfizer data set, completed after receiving fragmentary data dumps over two years, revealed substantial discrepancies between the reported and actual numbers of deaths among trial participants. Upon examining the deaths that occurred during the trial, we found a marked discrepancy between what was officially presented and the reality. Pfizer failed to disclose several vaccine-related deaths, including at least four instances where deaths occurred but were not reported in the core slides and briefing booklet presented to the FDA. This omission is particularly concerning as two of these deaths had been reported by family members to clinical trial sites before the cutoff date, obligating Pfizer to report them to the FDA by regulatory standards


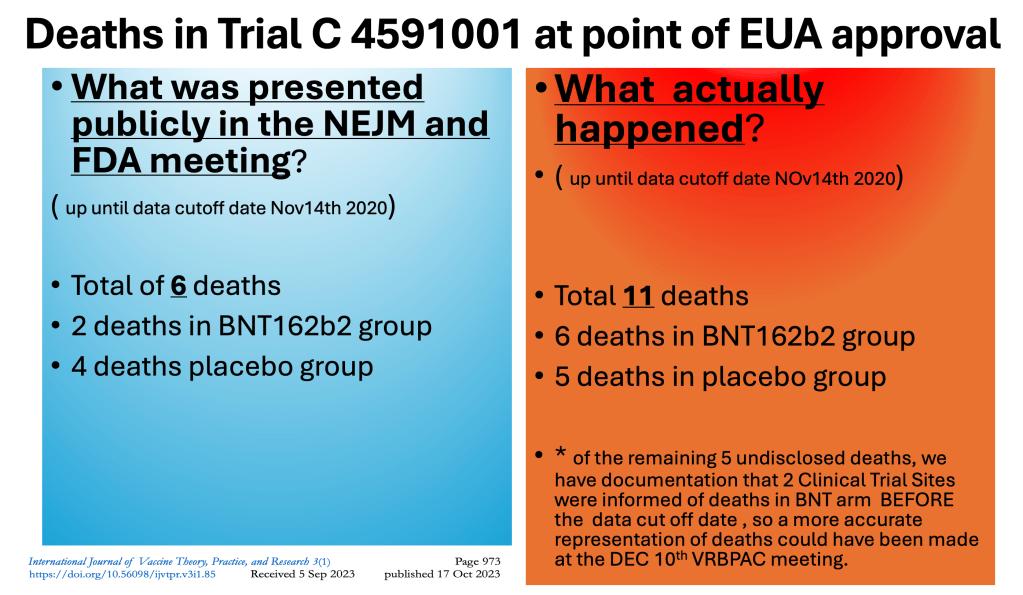
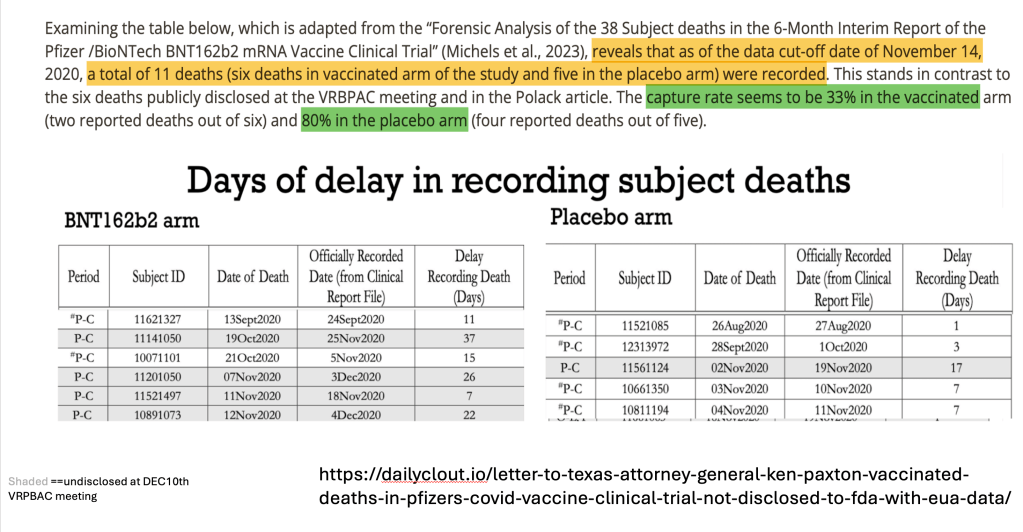
The study group not only experienced a higher number of deaths than the placebo group but also saw delays in the timing of those deaths compared to the placebo group.
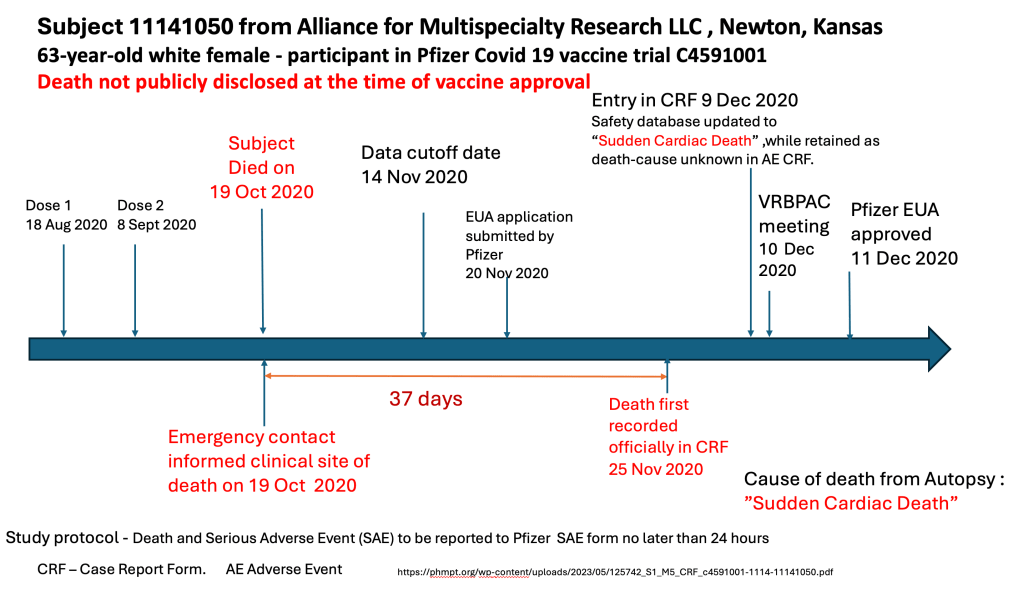
Kansas Sudden Death was reported to the trial site the day of death, but not recorded until 25 November
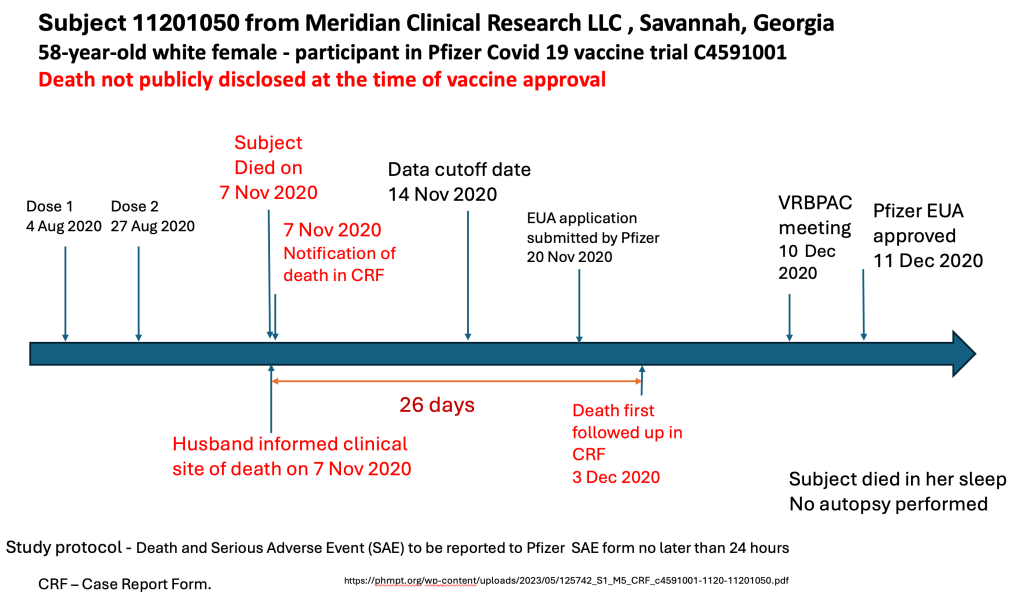
Georgia Sudden Death was reported on the day of death to Meridian, but not reported in the Pfizer files until 3 December
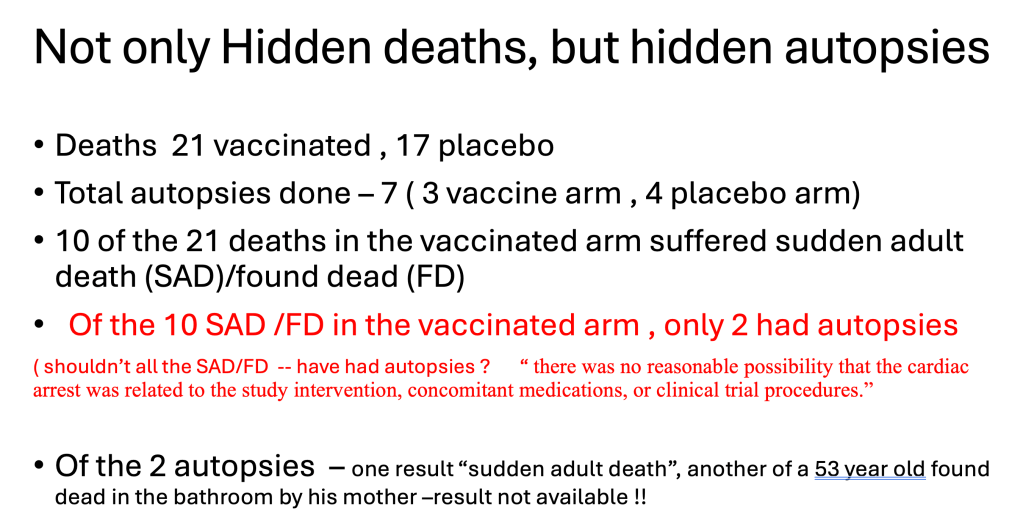
There was a dearth of autopsies of the sudden deaths during the clinical trial. 10 of the 21 deaths had ‘sudden adult death’, or ‘found dead’ classifications, which require an autopsy in normal circumstances.
As commented on by Nick Hulscher in his interview with Dr Kunadhasan,
“Pfizer failed to disclose four vaccine deaths in the core slides and briefing booklet. Two of these deaths had already been reported by family members to clinical trial sites before the cutoff date—meaning Pfizer was obligated by regulatory standards to report them to the FDA. They did not. From November 14, 2021 to the FDA VRBPAC meeting on Dec 10th, there were an additional 6 deaths. (2 additional vaccine deaths, 4 placebo). This means if anyone had asked Pfizer to update the panel on deaths not listed in the slides or briefing booklet, Pfizer should have disclosed 8 vaccine deaths and 9 placebo deaths. But no one asked.”

Also our initial interview on DailyClout following the release of our paper


